Explain the Difference Between K Kp and Q
So whats gonna happen is in order to reach equilibrium our concentrations are going to shift to the right to get Q closer to K. Explain the difference between K Kp and Q.
What Is The Relationship Between Kp And Kc Quora
C When Q K the reaction is at equilibrium.
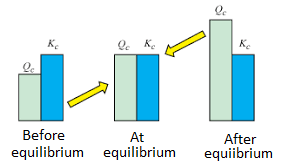
. Therefore when Q0 the reaction shifts to the right forward. Chemistry AP Edition 9th Edition Edit edition. Stack Exchange network consists of 180 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and build their careers.
We have step-by-step solutions for your textbooks written by Bartleby experts. Explain the difference between K K_p and Q. It is defined as the ratio of the concentrations of the.
K p is equilibrium constant used when equilibrium concentrations are expressed in atmospheric pressure and K c is equilibrium constant used when equilibrium concentrations are expressed in molarity. Q is a quantity that changes as a reaction system approaches equilibrium. The equilibrium constant of a reaction mixture is a number that expresses the ratio between the concentrations or pressure of products and reactants in that reaction mixture.
If Q equals to zero the reaction will shift forward to the right. K p And K c are the equilibrium constant of an ideal gaseous mixture. Think about the reaction between a very strong acid and water.
G Gibbs Free Energy K Equilibrium Constant and Q Reaction Quotient are related as follows. Kc and Kp are equilibrium constants. HCl is a much stronger acid than the H 3 O ion.
K is the numerical value of Q at the end of the reaction when equilibrium is reached. However the difference between the two constants is that Kc is defined by molar concentrations whereas Kp is defined by the partial pressures of the gasses inside a closed system. Students also viewed these Physical Chemistry questions.
Q Equals Zero. If Q is lower than K the system is composed of more reactants than products. Substituting these value in equilibrium constant expression equation 1 we have.
Therefore we can describe the relationship between Δ G and Kp for gases as follows. B When Q K the reaction goes to the left toward reactants. Kc and Kp are the equilibrium constants of gaseous mixtures.
For many general chemical reactions aA bB cC dD. Solutions for Chapter 13 Problem 17Q. You use Q when you are unsure if a reaction is at equilibrium.
Basically products over reactants with coefficients as exponents. Zumdahl Chapter 6 Problem 19E. Explain the difference between productivity as defined on p.
HClaq H 2 Ol H 3 O aq Cl-aq K a 10 6. G 0 and Qc Kc or Kp at equilibrium and no more changes in the concentration of the mixture. Therefore the reaction tends to form more products in order to keep the equilibrium.
So the value of Kc and Kp when the reactants and products are in their standard states is 1. Where a mole of reactant A. The reaction will proceed to form products.
If Q and K are equal then the reaction mixture is at an equilibrium. Explain the difference between K Kp and Q. KC involves concentrations of the reactants and products at equilibrium but Q uses the concentrations on their way to equilibrium.
K represents the equilibrium constant of the reaction in terms of concentrations. If Q K the reverse reaction is favoured the reaction moves from right to left until equilibrium is established. In Chapter 15 Chemical Equilibrium you learned that for gases Q Kp at equilibrium and as youve learned in this chapter Δ G 0 for a system at equilibrium.
The key difference between Kc and Kp is that Kc is the equilibrium constant given by the terms of concentration whereas Kp is the equilibrium. When this is the case and all values are given in pressures we use K p which is the equilibrium constant for pressure. KP KC RTcd- ab KP KC RTn.
Difference Between Reaction Quotient and Equilibrium Constant Definition. So Q we can put on our number line is somewhere around here. A When Q K the reaction goes to the right toward products.
And K is 145 so well say its somewhere around here. At equilibrium Q K the equilibrium constant for the reaction Q K is referred to as the equilibrium condition. Step 1 of 3.
So this is our Q and this is our K. Explain the difference between K Kp and Q. Textbook solution for Chemical Principles 8th Edition Steven S.
PA ART. It is important to understand the distinction between Q and K. If Q0 then Q is less than K.
Explain the difference between K Kp and Q. The table above provides us with the basis for understanding the difference between strong acids and weak acids. Explain the difference between K Kpand Q.
What is the difference between Q and Keq. K a 55. Explain the difference between K K p and Q.
An easy way to remember this relationship is by thinking once you have nothing the only thing left to do is to move forward. If Q K the forward reaction is favoured. This problem has been solved.
Solutions for Chapter 6 Problem 19E. We can see that Q is less than K on our number line. G 0 and Qc Kc or Kp at the start of the reaction.
K p And K c. The short answer which can be your mnemonic after you understand the issue is Q is what is. Q and Keq both have the same algebraic form.
2 where n cd ab the difference in the sums of the coefficients for the gaseous products and reactants. 100 5 ratings for this solution. Keq is what will be.
Find step-by-step Chemistry solutions and your answer to the following textbook question. So K c for Concentration and K p for Pressure.
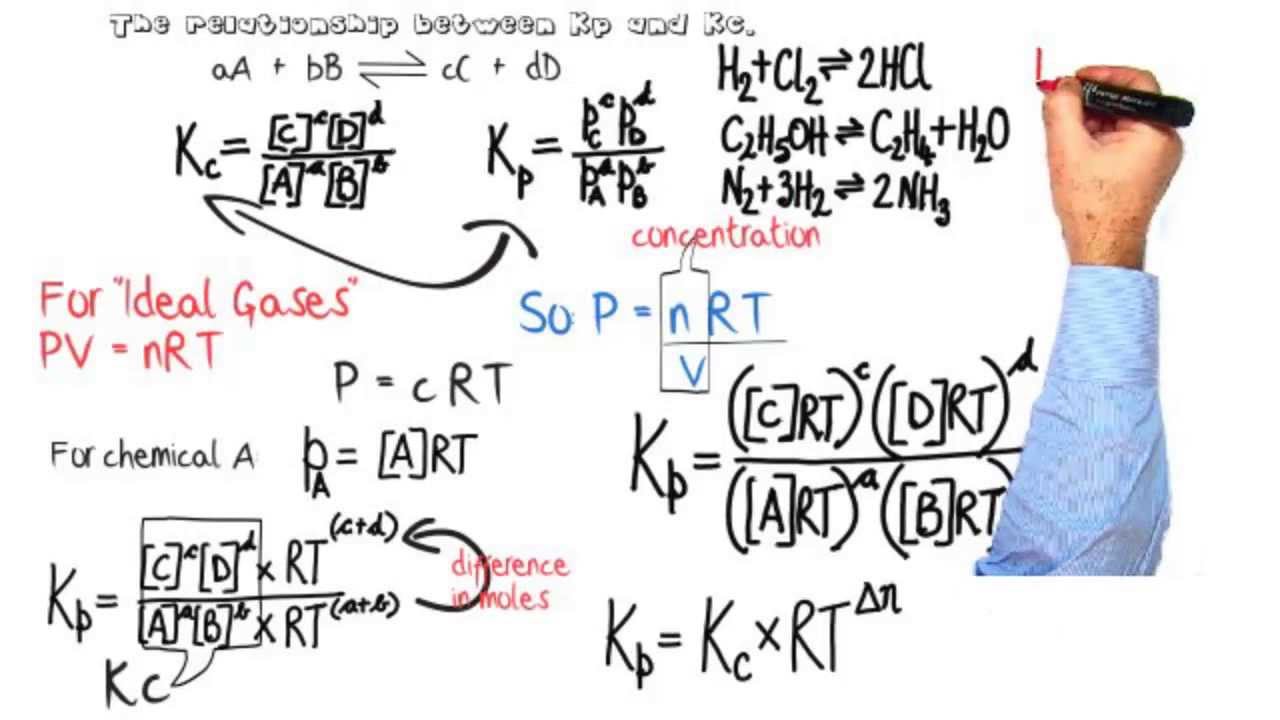
Equilibria Relationship Between Equilibrium Constants Kp Kc Youtube

No comments for "Explain the Difference Between K Kp and Q"
Post a Comment